The MACS® Matchmaker
Unlock new possibilities in your research today – because every target deserves its perfect match!
Experience a robust platform that delivers quantitative data on binder affinity, kinetics, and specificity in real samples.
This advanced biomolecular interaction system is not just another tool, it is designed to address your lab’s biggest challenges and redefine the way you approach drug discovery and interaction studies.
- Label-free measurements in crude media: in vivo-like environment and eliminate the hassle of pre-purification saving time.
- Simple assay development: Leverage our easy-to-use sensor functionalization protocol to set up your experiments faster than ever.
- Multiplexing for increased speed: Characterize 64 interactions simultaneously, significantly reducing experiment times.
- Assay automatization: Use the MACS® Sampler for fully unattended runs in combination with up to 2 x 384 well plates.
- Drift-free data: Experience robust and more reliable data with minimized artifacts by our patented focal molography technology.
- pM Sensitivity: Characterize even small proteins or peptides in complex biological matrices.
- Remote Instrument Control and Enhanced Connectivity: Control and access the instrument from any device any time.
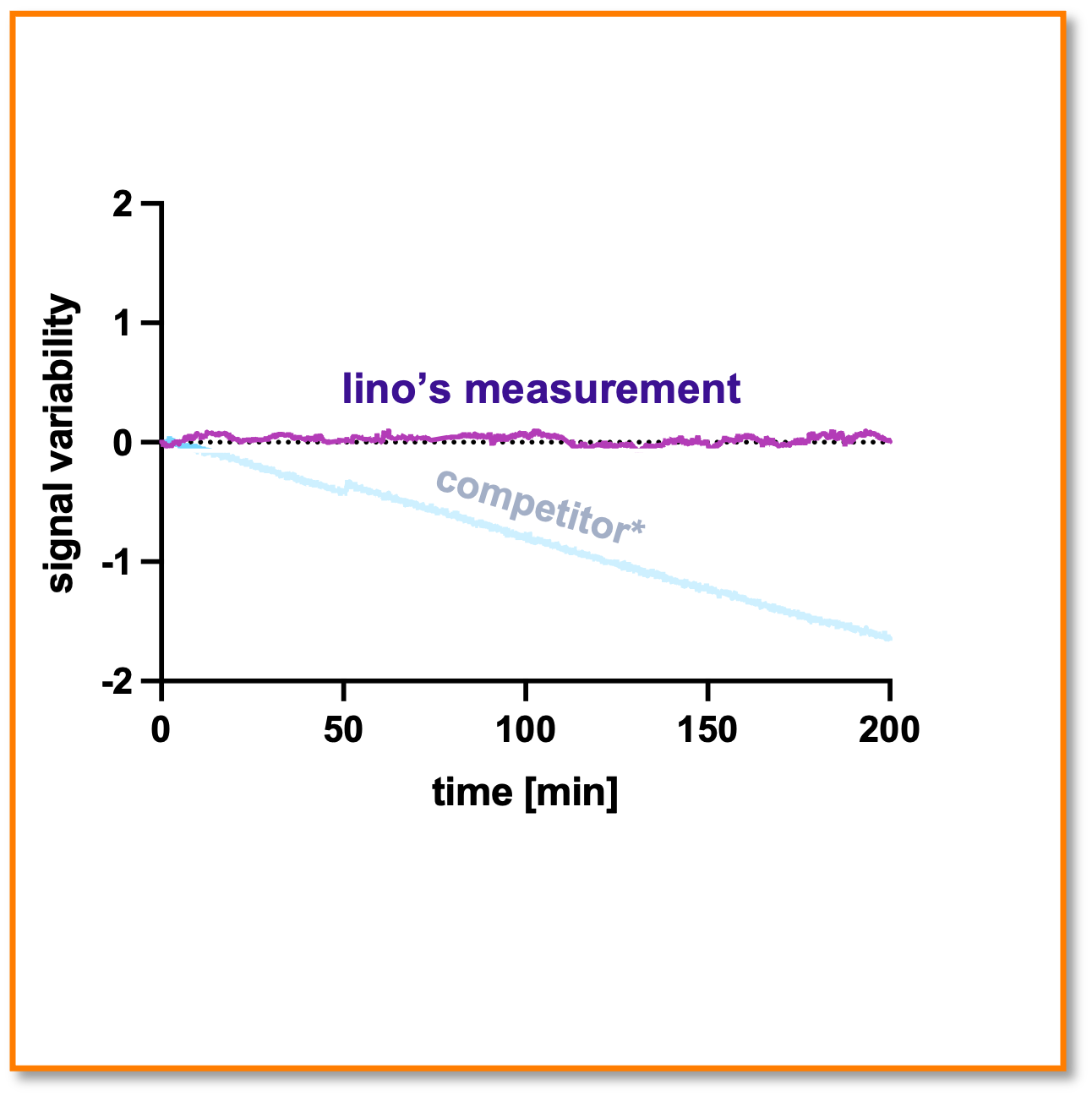
Drift-free baseline
The technology is essentially drift-free. Here, the signal against the latest versions of a competitor SPR instrument is shown over multiple hour (with reference channel subtraction). [Experiment was the signal stability under running buffer flow over multiple hours; no binding signal change is expected]
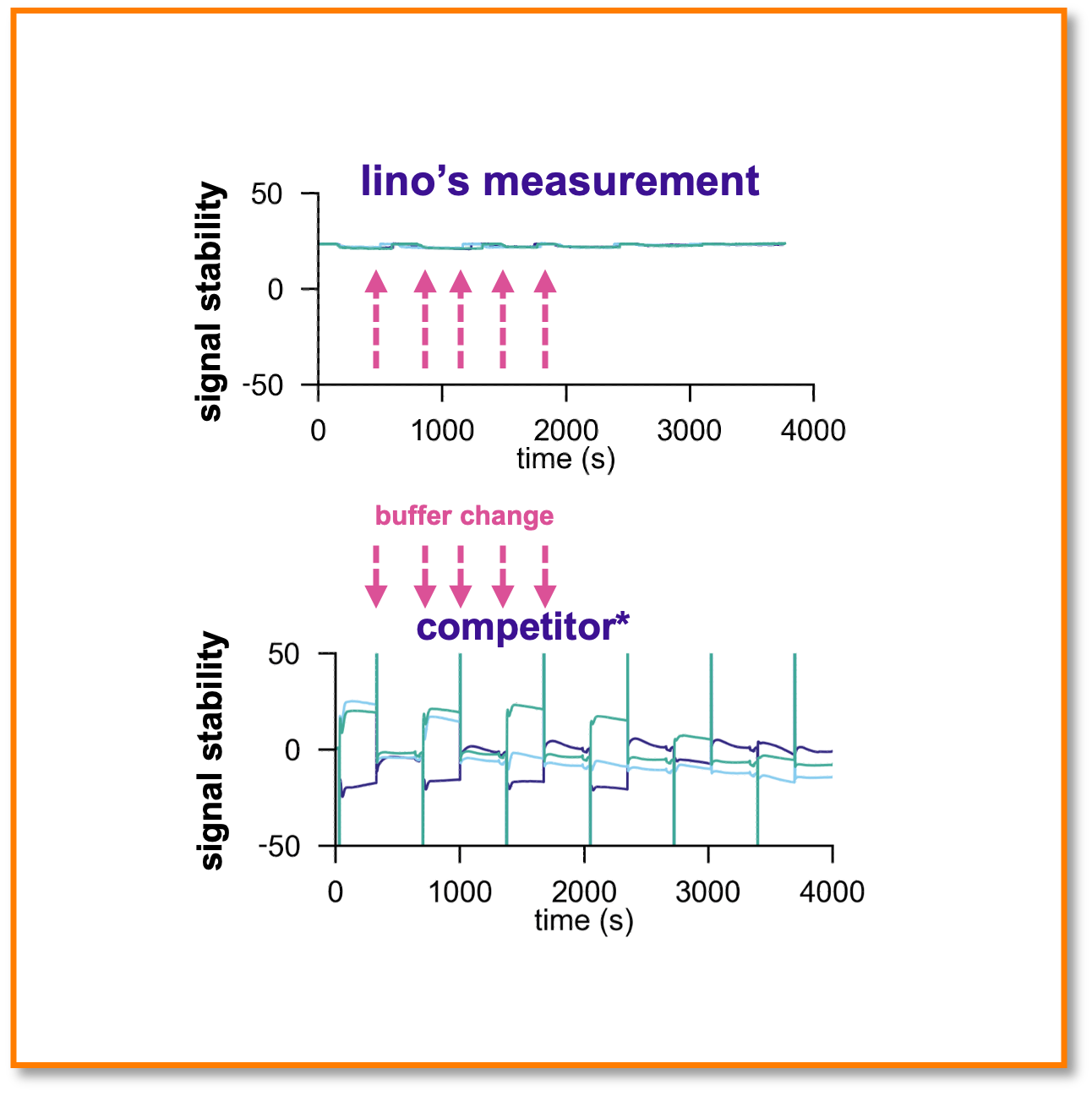
Lowest medium influence on the market
Molography has no artefacts from buffer changes during the measurement. Competitors (SPR) cannot tolerate buffer changes even after referencing. [Experiment was the injection of different glycerol concentrations; no binding signal is expected]
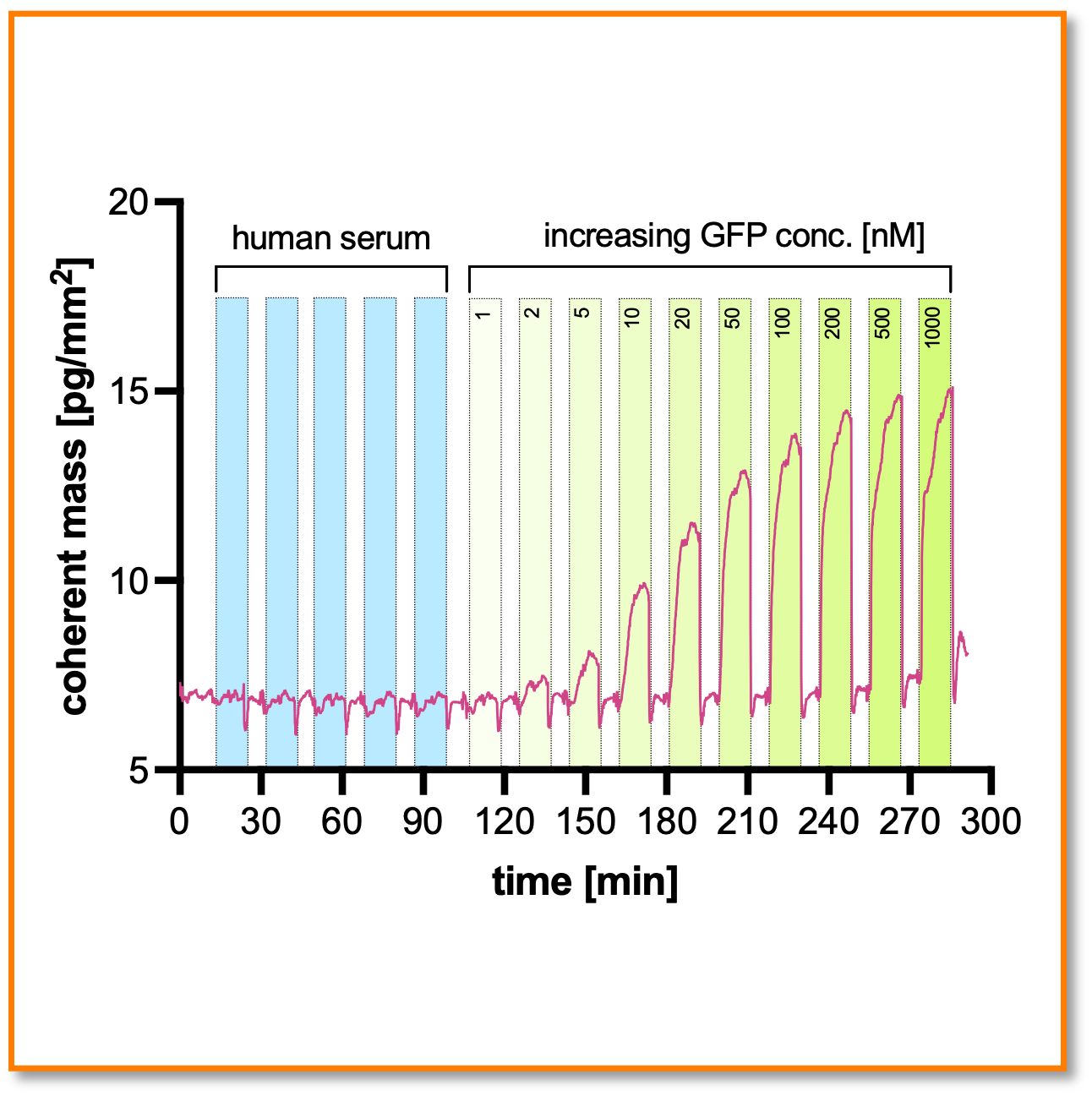
Makes crude samples look like buffer
Non-specific binding of serum proteins hardly generates a response. [Experiment detects spiked GFP in 50% human serum down to 2 nM concentrations]
The Matchmaker is powered by Focal Molography.
Focal molography is a biophysical technique that enables robust and sensitive detection of biomolecular interactions without the need for fluorescent labels. This method facilitates the analysis of these interactions within complex biological samples, such as blood sera, bioreactor fluids, or cell culture media.
The technique operates by diffracting laser light through a specialized two-dimensional nanopattern of molecular binding sites on a sensor chip, known as a mologram. This mologram functions as a focusing diffractive lens, directing light into a precise focal point. The intensity of this focused light correlates with the quantity of molecules bound to the mologram, thereby indicating the extent of biomolecular interactions.
A significant advantage of focal molography is its resilience against environmental noise, such as temperature fluctuations and nonspecific binding of off-target molecules. This robustness eliminates the need for temperature stabilization or sensor equilibration, making it a versatile tool for various applications.
Overall, focal molography expands the analytical capabilities for studying biomolecular interactions across a wide range of biological research and diagnostic applications.
Simplify your workflow and save valuable laboratory time with the fully automated MACS® Sampler.
Depending on your need you can combine the MACS® Matchmaker with the MACS® Sampler, a fully integrated automatization solution for your interaction studies.
- Sample format: 300 or 1800 µL vials, 96 or 384 well plates.
- Capacity: 2 positions for 48 vial tray or well plates.
- Throughput: Chracterize up to 1536 interactions per day*
- Flow path: One needle, one syringe pump
- Hands off: Up to 4 days unattended run time
- Biocompatibility: metal-free flow path for samples injection via biocompatible sample needle, valves, and tubing.
- Cooling: Sample compartment can be chilled at 4 °C for optimal storage.
- Software: seamless integration into the MACS® Matchmaker software.
*with DNA directed immobilization chemistry in batches of 64 immobilized compounds against one target.

Technical Specifications
| Association constant range | kon = 103-108 M-1 s-1 |
| Dissociation constant range | koff = 10-6- 1 s-1 |
| Noise and Drift (over 20 mins) at 5 pg/mm2 | 0.05 pg/mm2 (roughly 0.05 RU) |
| Noise and Drift (over 20 mins) at 50 pg/mm2 | 0.2 pg/mm2 (roughly 0.2 RU) |
| Lower limit molecular weight (Analyte) | 2 kDa |
| DMSO (10 %) spike response | 1-2 pg/mm2 (1000 times lower than in SPR) |
| Readout Frequency | 0.5 Hz |
| Analysis temperature range (without chiller option) | 25 - 60 °C |
| Analysis temperature range (with chiller option) | 15 - 60 °C |
| Compatible Crude Sample Types | Blood, Plasma, Serum, Cell lysate, Cell culture supernatant, Urine, CSF, Interstitial fluid |
Imaging capabilities
| Image resolution | 5 μm |
| Numerical Aperture | 0.1 NA |
| Field of view | 7 x 8 mm |
| Fluorescence Excitation wavelength | 660 nm |
| Compatible Fluorophores | Cy5, Alexa Fluor 660 and 680, Atto 647N, DyLight 660, APC, Nile Blue, IRDye 680 |
| Application | Cell counting, Calcium signaling assays, hypothesis validation |
| Illumination | Waveguide total internal reflection illumination (only first 100-200 nm above the surface are illuminated. |
Fluidics and Sample handling
| Sample Capacity | 2x microtiter plates (96 or 384 well, standard and deep well) or vial racks (12 positions (10 mL) or 48 positions (300 μl or 1.5 ml) |
| Degasser | Built-in |
| Number of running buffers | 1 |
| Flow rates | 6 - 1000 μl/min |
| Fluidic heads | Open pipetting adapter, Handpipetting Adapter, 6 channel adapter |
| Flow cells | Single flow chamber, 4 and 6 parallel flow chambers |
| Channel referencing | Not required thanks to self-referencing principle |
| Flow chamber height | 100 μm |
| Injection Volume | 50 - 500 μl, 100 μl typical (20 μl with handpipetting adaptor) |
| Sample Storage Temperature | 4 - 20 °C or ambient |
| Unattended run time | 72 h |
Software and data evaluation
| Information provided | Kinetic and affinity data (kon, koff, KD), surface and fluorescence images |
| Data analysis | Full automated data analysis for standard workflows, Jupyterhub (Python) integration for advanced analysis |
| Data Visualization and export | Interactive html graphs, csv, excel and pdf |
| Remote operation | Yes, integrated PC |
| IoT integration | LAN, WLAN |
| Control and evaluation hardware | Desktop, Labtop, Tablet (Modern web UI) |
Accessories
| Chilling module |
| MACS Sampler |
| Incubation chamber (CO2, Humidity Control) |
